(a) Explain why it is not advisable to sterilize a clinical thermometer in boiling water at normal atmospheric pressure. (b) State the effect of an increase...
Question 1 Report
(a) Explain why it is not advisable to sterilize a clinical thermometer in boiling water at normal atmospheric pressure.
(b) State the effect of an increase in pressure on the
(i) boiling point; and
(ii) melting point of water.
(c) Diagram: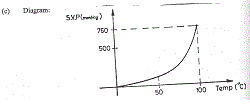
The graph shown above is that of the saturated vapour pressure (s.v.p.) of water against temperature.
Pure water is known to boil at 100\(^o\)C and at an atmospheric pressure of 760 mmHg. What general conclusion can be drawn from the information given above?
d) A thread of mercury of length 20 cm is used to trap some air in a capillary tube with uniform cross-sectional area and closed at one end. With the tube vertical and the open end uppermost, the length of the trapped air column is 15cm. Calculate the length of the air column when the tube is held:
i) horizontally;
ii) vertically with the open end underneath. [Atmospheric pressure = 76 cmHg ]
Download The App On Google Playstore
Everything you need to excel in JAMB, WAEC & NECO
